Calliditas – pioneering new treatments for rare diseases
Calliditas Therapeutics leverages scientific expertise and disease-specific insights to improve the lives of patients. We are a commercial-stage biopharma company that researches, develops and commercializes novel therapies that address significant unmet needs in rare diseases. We are committed to expanding treatment options and establishing new standards of care for patients with rare diseases, reflected by our pipeline of innovative medicines that target unmet medical needs.
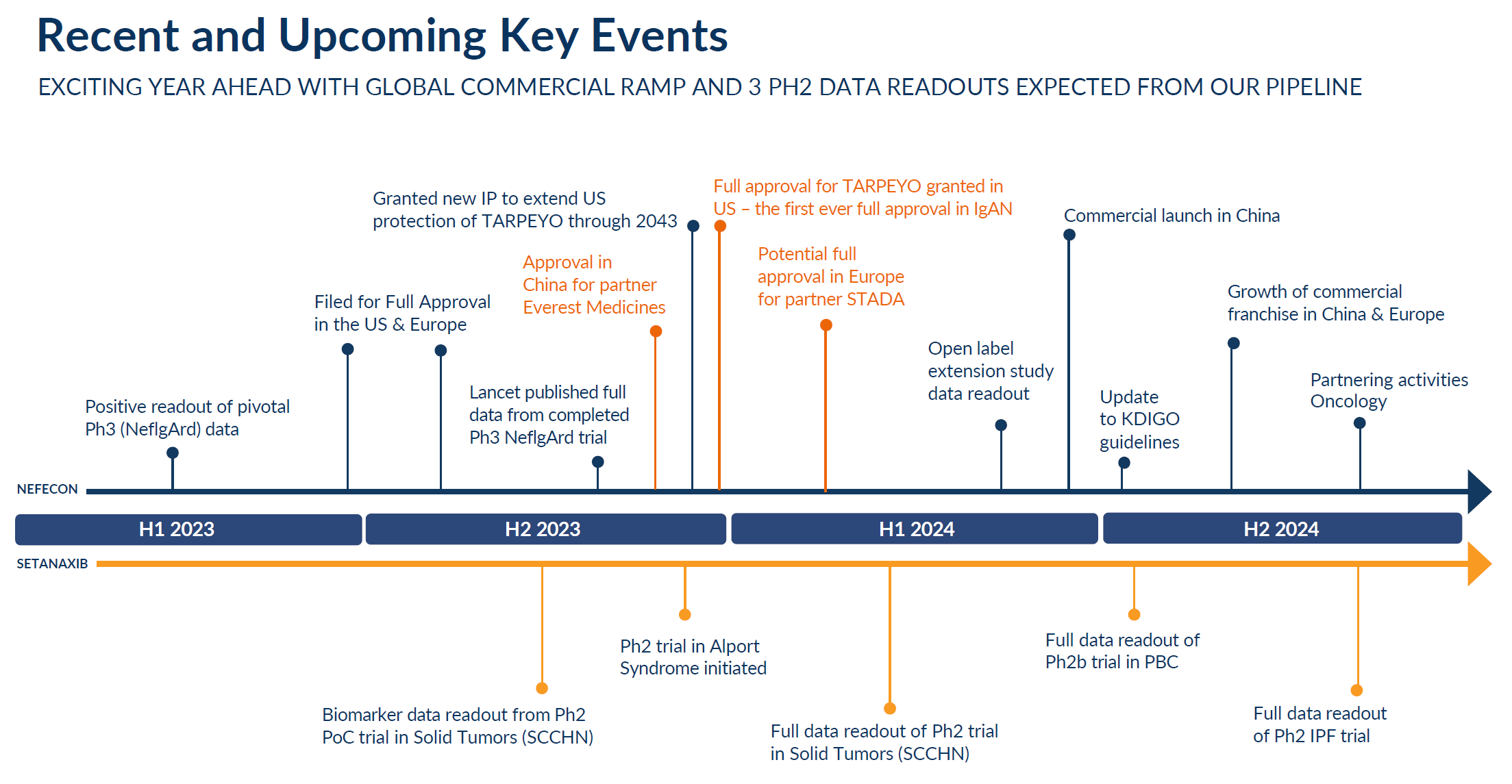
Our lead product provides provides a treatment option that we believe is disease-modifying for IgA nephropathy (IgAN) – also known as Berger’s Disease – a progressive autoimmune disease of the kidney that for many patients leads to end-stage renal disease (ESRD), requiring dialysis or organ transplantation. This drug product, developed under the name Nefecon®, was granted accelerated approval by the FDA in 2021, and is today marketed in the US under the brand name TARPEYO®. It has also been granted conditional marketing authorisation by the European Commission under the brand name Kinpeygo® in the European Economic Area (EEA) and in the UK. TARPEYO and Kinpeygo are currently being reviewed by the FDA and EMA for full approval, with the US PDUFA date set for December 20, 2023.
Nefecon has also been approved in China and Macau and is being reviewed by regulators in Singapore, Hong Kong and South Korea. Calliditas has also recently entered into a partnership to develop and commercialise Nefecon in Japan.
IgA nephropathy is the largest of the glomerular nephritis diseases, so the market potential for Nefecon is substantial, as evidenced by out-licensing deals with potential payments exceeding USD 300 million, encompassing upfront payments and predefined milestones, as well as ongoing royalty obligations.
Our late-stage pipeline is based on a first-in-class platform of NOX inhibitors. Our lead compound, setanaxib, inhibits enzymes involved in inflammation and fibrosis pathways and is the first drug of this class to reach the clinical stage. Setanaxib is currently undergoing clinical trials targeting rare diseases characterized by inflammation and fibrosis, including IPF and PBC, and Calliditas is also planning to launch a trial with setanaxib in Alport syndrome. Additionally, based on promising preclinical findings, we are conducting a proof-of-concept trial in head and neck cancer to further support the mode of action of this drug class.
While our headquarters are in Stockholm, Sweden, we maintain a significant presence in the United States, with offices in New York and New Jersey. We also have offices in France and Switzerland, where our discovery team is based. Calliditas Therapeutics ordinary shares were listed on NASDAQ Stockholm in 2018 (CALTX) and subsequently American Depositary shares representing our ordinary shares were listed on the NASDAQ Global Select Market in the United States in 2020 (CALT).
Our values
AGILITY
We are flexible and able to rapidly pivot and adapt to changing situations and requirements.
Integrity
We take responsibility for our actions and hold ourselves to the highest ethical standards, guided by our moral principles to make the right decisions.
Expertise
We leverage our strong internal experience and competencies while complementing our strengths through knowledge sharing and external collaborations as needed.
Pioneer
We explore novel approaches and empower each other to find new ways of operating in a compliant, innovative and pragmatic manner.