
Primary Biliary Cholangitis (PBC)
PBC is an idiopathic, progressive, and chronic autoimmune disease of the liver. It causes a cycle of immune injury to biliary epithelial cells, resulting in inflammatory and liver disorders. PBC is an orphan disease affecting ~140,000 patients in the United States, with women making up about 90% of all cases.
Current therapies are limited, and mainly target cholestasis – anticholestatic drugs and obeticholic acid, 1L and 2L respectively 45% of patients fail to respond/intolerant to UDCA. Disabling, clinically meaningful, symptoms such as fatigue are not addressed by current therapies. Further, there are no approved therapies for PBC that specifically target fibrosis of the liver.
Setanaxib addresses elements of PBC that no approved, or phase 3 drugs target
A novel anti-fibrotic agent like setanaxib could delay disease progression and obviate transplant. Setanaxib is differentiated in its positive impact on important QoL measures such as fatigue.
Up to 80% of PBC patients suffer from chronic fatigue which meaningfully impacts QoL.
Setanaxib Directly Inhibits Fibrogenesis in the Liver
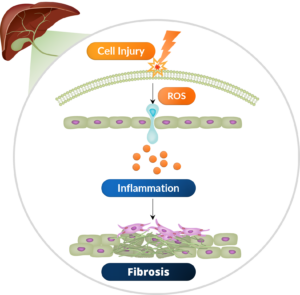 The pathogenesis of PBC is a cycle of inflammation and fibrosis enabled by ros-driven cell injury.
The pathogenesis of PBC is a cycle of inflammation and fibrosis enabled by ros-driven cell injury.
PBC patients undergo immune injury to their biliary epithelial cells, which results in an inter-dependent chronic cycle of cholestasis and fibrosis in the bile ducts and surrounding tissue.
This results in the generation of excess ROS, resulting in cell death and a further cascade of inflammatory signaling which sets up the cycle of damage to the integrity of the bile ducts.
Inflammation is the primer of fibrosis, which is the cellular ‘reparative response’ to cell injury.
Fibroblasts in the surrounding stromal space become activated myofibroblasts and secrete fibrotic scar tissue.
The persistent biliary inflammation fuels the stimulation of fibrosis as a reparative response to the tissue, and as a result, there is excessive deposition of fibrotic tissue in the portal space.
Statistically Significant Changes in Biomarkers in Legacy Ph2 Study
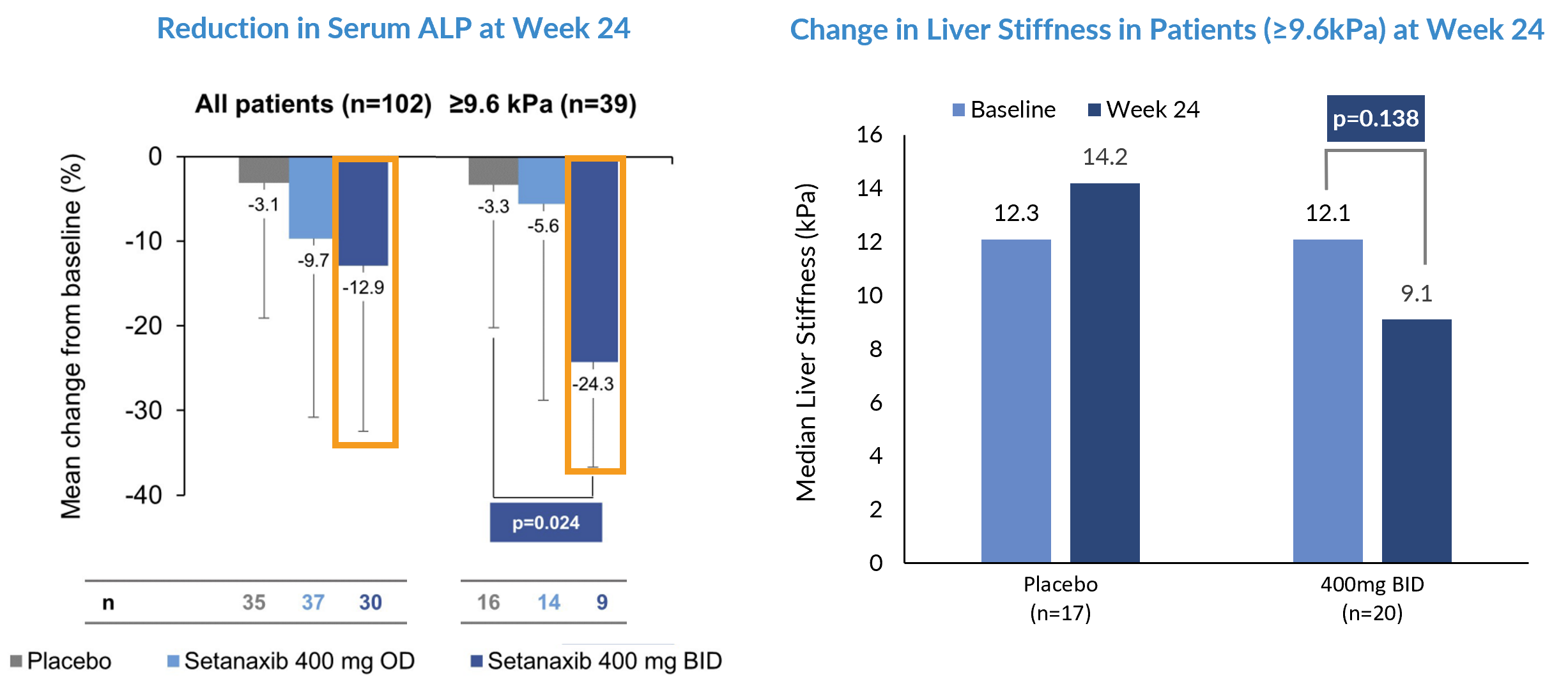
Signal of statistically significant mean ALP reduction in more severe PBC patients underpins Ph2b rationale.
While the primary endpoint of GGT blood test in the legacy Ph2a RCT (NCT03226067) was not met, the statistically significant improvements in ALP and liver stiffness with the 400mg BID dose informed the rationale for using higher doses in the Ph2b study.
The sub-group of patients with liver stiffness ≥9.6kPa demonstrated a statistically significant mean reduction in ALP* from baseline (p=0.024) vs. placebo and a difference of ~3kPa in mean liver stiffness which represents a 1-point fibrosis score reduction.
Significant Benefit on Clinically Meaningful Quality of Life Measures
Legacy Ph2 showed highly statistically significant improvement in fatigue – not addressed by existing therapies
At 400mg BID setanaxib resulted in highly statistically significant (p<0.005) improvement on fatigue, as well as emotional and social disease aspects in all patients at week 24.
Current studies suggest that up to 50% of patients with PBC experience clinically significant fatigue and it is more common in younger patients presenting with the disease.
Phase 2b in Primary Biliary Cholangitis
RCT Ongoing (NCT05014672) with ODD granted for Setanaxib by FDA & EMA and FTD by FDA
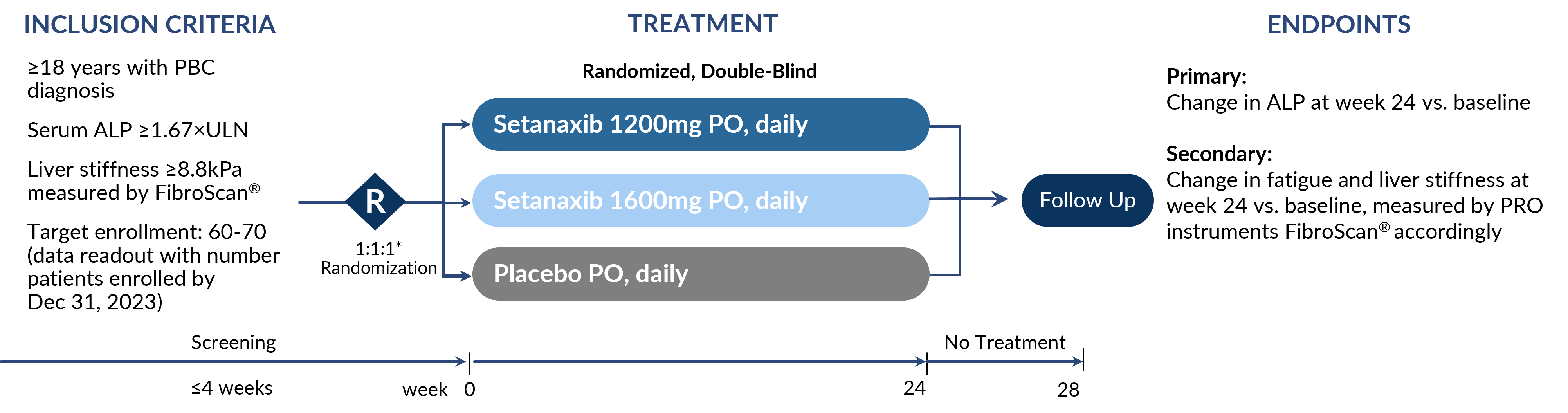
Data to inform development in PBC, PSC, or more broadly around fatigue.
Full data readout expected mid-2024.