
Setanaxib Platform Development
Setanaxib is a pipeline in a product in development for fibrotic rare diseases and solid tumors.
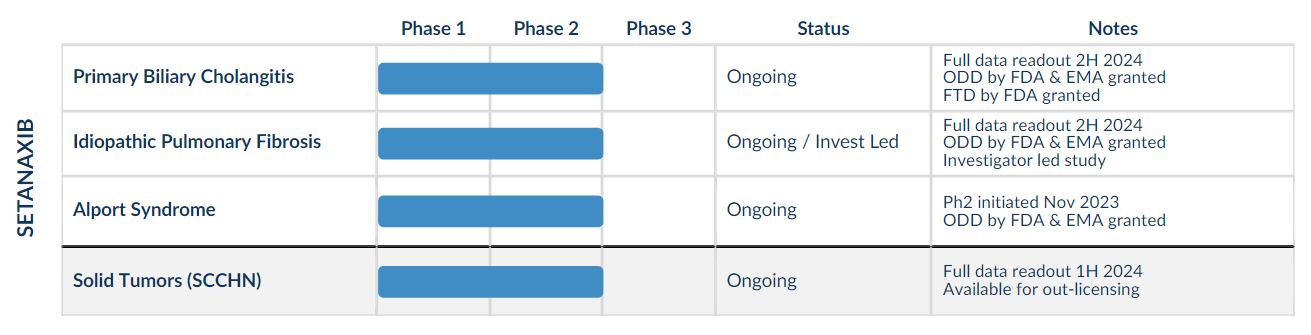
Calliditas holds global rights to setanaxib in all indications. The asset boasts an extensive safety dataset with >320 subjects exposed to setanaxib in completed Ph1 and Ph2 clinical trials.
We are looking forward to 3x Phase 2 data readouts in 2024 – Solid Tumors (SCCHN), PBC and IPF.